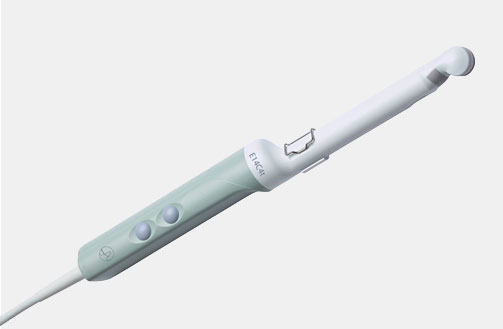
E14C4t (9018) Prostate Triplane Transducer
9018/ View Product Page
Follow local regulations for minimum reprocessing. Check table 4 on page 29 of the Care and Cleaning Guide.
Follow product manufacturer’s instructions and do not exceed transducer specified limits. See Care and Cleaning Guide for more information.
Applicable Documents:
Cleaning Agent
Compatibility
Compatibility Type
Comments
3E-Zyme
Yes
Validated
—
Bodedex forte
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
CIDEZYME XTRA Multi-Enzymatic Detergent/ CIDEZYME GL Enzymatic Detergent
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
E14C4t (9018) Prostate Triplane Transducer
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
Gigazyme
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
Intercept Wipes/Intercept Detergent
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
Korsolex Endo-Cleaner 0,5%
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
MATRIX Biofilm Remover
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
Neodisher MediClean Forte
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
pH neutral (pH 6-8), non-corrosive cleaning products intended for medical devices
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
Prolystica 2x concentration. Enzymatic
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
Revital-Ox Bedside Complete/2X Concentrate Enzymatic Detergent/Enzymatic Detergents
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
Sekusept MultiEnzyme P
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
Suma Med Enzyme
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
Medivators Advantage PlusIntercept (detergent), Rapicide PA Disinfectantflush: 70% isopropyl alcohol (Automated disinfection)
Yes
Validated
Rapicide PA disinfectant is not listed as FDA-cleared high-level disinfectant.
CIVCO UltrOx HLD
Yes
Validated
—
Tristel Fuse for Instruments (Tristel Fuse for Stella)
Yes
Validated
—
Revital-Ox Resert/Resert XL HLD
Yes
Validated
—
Ethanol 70% (wiping)
Yes
Validated
—
Korsolex Basic
Yes
Validated
—
Accel Prevention (wipes, ready-to-use liquid, concentrate)
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
Adaspor Single Shot (Automated disinfection)
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
Antigermix S1 (Automated disinfection)
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
Astra VR (with approved disinfectant)
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
Bomix Plus
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
Cavi Wipes/CaviCide
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
Cidex OPA
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
Cleanisept Wipes Forte
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
E14C4t (9018) Prostate Triplane Transducer
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
E14C4t (9018) Prostate Triplane Transducer
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
Gigasept FF
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
Glutaraldehyde 2% – 3.4%
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
Incidin OxyFoam/Incidin OxyWipe S
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
Isopropanol 70% (wiping)
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
Korsolex Endo Disinfectant 1%/Korsolex Extra
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
Meliseptol Foam
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
Meliseptol Wipes Sensitive
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
Metricide OPA Plus
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
Mikrobac Tissues
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
Mikrozoid AF Liquid
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
Neodisher Endo Sept GA
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
Neodisher Endo SEPT PAC/Neodisher Septo PAC
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
Neodisher Septo DN
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
Nu-Cidex
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
OPAL
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
Rapicide/Rapicide OPA/28
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
Rely+On Perasafe
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
Sani Cloth Plus Wipes/Sani Cloth Super Wipes
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
Sekusept Aktiv
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
Steranios 2%, 2% N.G., 2% E.C.S
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
Thermosept PAA
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
Tristel Duo for Ultrasound/Tristel Trio Wipe System
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
Trophon EPR / Trophon 2
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.Fit transducer completely above line mark.
STERIS System 1, 1E, 1 Plus and 1 Express
Yes
Validated
—
STERIS V-Pro 1 Plus, V-Pro 60 Non lumen cycle
Yes
Validated
—
STERIS V-Pro maX Non lumen cycle or Flexible cycle
Yes
Validated
—
Sterrad NX and 100NX Standard cycle
Yes
Validated
—
Sterrad 100 NX Express Cycle
Yes
Validated
—
Sterrad 100S One cycle only (USA), Short cycle (rest of the world)
Yes
Validated
—
Sterrad 200 Short cycle
Yes
Validated
—
Matachana 130LF, Webeco FA90, Webeco FA95 (60°C cycle)
Yes
Validated
Use a suitable connector lid and valve from the system manufacturer. See page 45 in the Care and Cleaning guide.
E14C4t (9018) Prostate Triplane Transducer
No
—
—
RENO-20/RENO-30/RENO-D50: ECO Cycle
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—
RENO-S90, RENO-S130, RENO-S130D: ECO cycle and Non Lumen cycle
Yes
Material Compatible
a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.—

